Select Publications from the Duong Research Group
Advances in nanobody multimerization and multispecificity: from in vivo assembly to in vitro production (2025). Biochemical Society Transactions.
Mohammed Al-Seragi, Yilun Chen, Franck Duong van Hoa.
NANOBODIES® (Nbs) are valuable in therapy, diagnostics, and industry due to their small size, enabling them to target areas inaccessible to regular antibodies. While Nbs typically exhibit strong specificity, combining multiple Nbs can enhance binding strength. Recent studies have focused on linking multiple Nb clones, using methods like flexible linkers, antibody domains, self-assembling coils, chemical bonding, and clustering sequences. Successful Nb combinations have been demonstrated in tests and virus neutralization. This review outlines techniques for creating combined Nbs and discusses their uses, benefits, and limitations.
Peptidisc-assisted hydrophobic clustering toward the production of multimeric and multispecific Nanobody proteins. (2025). Biochemistry.
Yilun Chen and Duong van Hoa F. Multimerization enhances protein stability, variety, and effectiveness through methods like chemical links, self-assembly, and cross-linking. We introduce a new application using peptidisc to stabilize proteins via hydrophobic interactions, effectively used with nanobodies (nAbs), which are cheaper to produce. For example, a nAb targeting green fluorescent protein (GFP) created "polybodies" with stronger binding, showcasing multimerization benefits. A nAb against human serum albumin also yielded improved results, confirming stronger binding advantages. This method facilitates bispecific polybodies and GFP variants, demonstrating the design flexibility for multifunctional proteins. Peptidisc-assisted clustering marks a significant advancement in protein multimerization with applications in life sciences.
Membrane mimetic thermal proteome profiling (MM-TPP) towards mapping membrane protein-ligand dynamics (2024). eLIFE.
Jandu RS, Al-Seragi M, Aoki H, Babu M, Duong van Hoa F. Integral membrane proteins (IMPs) are key targets for small-molecule drugs, yet reliable methods to assess their interactions without detergents are lacking. We present the Peptidisc membrane mimetic (MM) to address this. This study combines the Peptidisc method with thermal proteome profiling (TPP) to analyze membrane protein-ligand interactions in a detergent-free setting. Using a library from whole mouse liver, we investigate ATP and orthovanadate's effects on IMP stability, focusing on transporters and receptors. MM-TPP also detects stability changes caused by ATP by-products, confirming non-standard ATP binders through computational methods. This new platform enhances our understanding of ligand effects and membrane protein stability amid small molecule interactions.
Sensitive profiling of mouse liver membrane proteome dysregulation following a high-fat and alcohol diet treatment (2024). PROTEOMICS.
Antony F, Brough Z, Orangi M, Al-Seragi M, Aoki M, Babu M, Duong van Hoa F.
Alcohol use and high-fat diets harm liver function. While research has focused on protein changes in alcoholic liver disease (ALD) and metabolic liver disease (MASLD), membrane proteins have been less studied. Traditional methods often miss key membrane details. This study employed the Peptidisc method to analyze liver membrane proteins. In mice on a high-fat diet with ethanol, we identified over 1,500 liver proteins, half of which had transmembrane segments. We found 106 integral membrane proteins altered compared to untreated samples. Gene analysis showed disruptions in lipid metabolism, cell adhesion, foreign substance processing, and mitochondrial membrane formation. Pathways for cholesterol and bile acid transport were affected, suggesting an adaptive response to liver fat buildup. Our research underscores the significance of membrane proteins in disease and highlights potential targets for treatment or diagnosis.
From bottom-up to cell surface proteomics: detergents or no detergents, that is the question (2024). Biochemical Society Transactions.
Zora Brough, Zhiyu Zhao and Franck Duong van Hoa
Understanding membrane protein expression is vital for cell differentiation, disease characterization, biomarker identification, and therapeutic development. While bottom-up proteomics surveys the membrane proteome, the low abundance and hydrophobicity of these proteins complicate sample preparation. Conventional extraction methods use detergents, which, while preventing aggregation, become contaminants that interfere with analysis. Various detergent removal strategies, including FASP, SP3, S-Trap, and membrane mimetics, exist to address this. This review examines each method's fundamentals, applications, advantages, and limitations, offering insights into their effectiveness in membrane protein research.
Capture of endogenous lipids in peptidiscs and effect on protein stability and activity (2024). iScience.
Jandu RS, Yu H, Zhao Z, Le HT, Kim S, Huan T and Duong van Hoa F.
Protein-lipid interactions are less understood than protein-protein and protein-nucleic acid interactions, primarily because membrane proteins (MPs) are insoluble in water. Detergents used for solubility can disrupt MPs and extract vital lipids. Recently developed membrane mimetics, like peptidisc, permit the isolation of MPs under various lipid conditions, influencing their activity and stability. Peptidisc also enables the incorporation of specific lipids to modify the protein environment and study related interactions. This research offers early insights into protein-lipid interactions, paving the way for further exploration of lipid influences on membrane protein functions.
Capture of the Mouse Organ Membrane Proteome Specificity in Peptidisc Libraries (2024). Journal of Proteome Research.
Frank Antony, Zora Brough, Zhiyu Zhao, and Franck Duong van Hoa
Unveiling Membrane Proteins
Cell surface proteins are vital for bodily processes and linked to diseases, yet they are challenging to study due to their low abundance and hydrophobicity. Our team has developed peptidiscs that mimic cell membranes, facilitating research using advanced techniques. Recently, we identified hundreds of cell-surface proteins in various mouse organs, enhancing our understanding of their functions. This research could advance biomarker exploration and other biomedical studies by revealing insights into these proteins.
A Peptidisc-based Survey of the Plasma Membrane Proteome of a Mammalian Cell (2023). Molecular and Cellular Proteomics.
Zhiyu Zhao, Arshdeep Khurana, John W. Young, Keeley G. Hewton, Zora Brough, Tianshuang Zhong, Seth J. Parker, Franck Duong van Hoa
Membrane proteins are crucial at the cell surface, with their malfunction linked to various diseases. Evaluating the plasma-membrane proteome is vital for cell biology and identifying biomarkers and therapeutic targets. However, their low abundance compared to soluble proteins complicates characterization, even with advanced proteomics. This study utilizes the peptidisc membrane mimetic to purify the cell membrane proteome. In the HeLa cell line, the peptidisc captures over 500 integral membrane proteins, half associated with the plasma membrane. The method is also applied to compare two pancreatic cell lines, allowing purification of proteins in a stabilized water-soluble state.
Development of a Method Combining Peptidiscs and Proteomics to Identify, Stabilize, and Purify a Detergent-Sensitive Membrane Protein Assembly (2022). Journal of Proteome Research
John William Young, Irvinder Singh Wason, Zhiyu Zhao, Sunyoung Kim, Hiroyuki Aoki, Sadhna Phanse, David G Rattray, Leonard J Foster, Mohan Babu, Franck Duong van Hoa
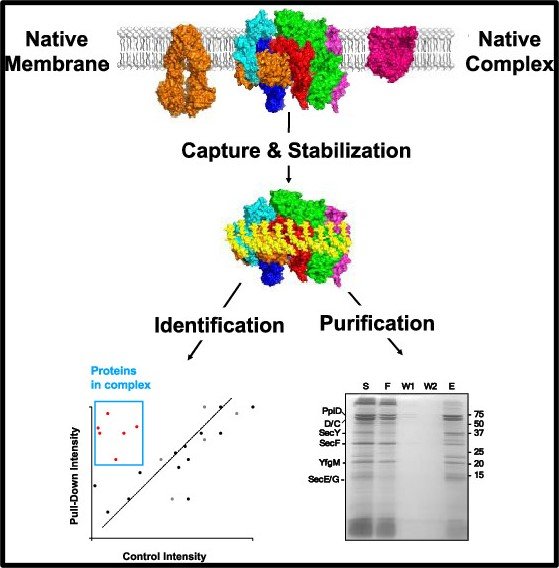
The peptidisc membrane mimetic allows the reconstitution of bacterial membrane proteomes into water-soluble, detergent-free particles called peptidisc libraries. We present a method combining peptidisc libraries and chromosomal-level gene tagging with affinity purification and mass spectrometry (AP/MS) to stabilize and identify fragile membrane protein complexes at native levels. This approach addresses artifacts from bait overproduction and protein complex dissociation due to detergent exposure. Using Escherichia coli Sec system, we identify the HMD complex, an expanded translocon with nine integral membrane subunits, stable in peptidiscs but dissociating in detergents. Based on this proteomic information, we establish a method for purifying the HMD complex with minimal dissociation. These findings demonstrate the effectiveness of peptidiscs and AP/MS in stabilizing fragile membrane protein assemblies.
A Dual Detergent Strategy to Capture a Bacterial Outer Membrane Proteome in Peptidiscs for Characterization by Mass Spectrometry and Binding Assays (2022). Journal of Proteome Research
John William Young, Zhiyu Zhao, Irvinder Singh Wason, and Franck Duong van Hoa
Characterization of the bacterial outer membrane remain challenging, however, and low throughput, due to the high hydrophobicity and relatively low abundance of this cell compartment. Here we adapt our peptidisc-based method to selectively isolate the outer membrane proteome before analysis by mass spectrometry. Using a dual detergent membrane solubilization approach, followed by protein purification in peptidiscs, we capture over 70 outer membrane proteins, including 26 integral β-barrels and 26 lipoproteins. Many of these proteins are present at high peptide intensities, indicative of a high abundance in the library sample. We further show that the isolated outer membrane proteome can be employed in downstream ligand-binding assays.
Nanodisc-Based Proteomics Identify Caj1 as an Hsp40 with Affinity for Phosphatidic Acid Lipids (2021). Journal of Proteome Research
Xiao X. Zhang, John William Young, Leonard J. Foster, and Franck Duong
The protein–membrane interactions are difficult to characterize due to their often-transient nature as well as phospholipids’ poor solubility in aqueous solution. Here, we employ nanodiscs along with quantitative proteomics to identify lipid-binding proteins in Saccharomyces cerevisiae. Using nanodiscs reconstituted with yeast total lipid extracts or only phosphatidylethanolamine (PE-nanodiscs), we capture several known membrane-interacting proteins, including the Rab GTPases Sec4 and Ypt1, which play key roles in vesicle trafficking. Utilizing PE-nanodiscs enriched with phosphatidic acid (PEPA-nanodiscs), we specifically capture a member of the Hsp40/J-protein family, Caj1, whose function has recently been linked to membrane protein quality control. We show that the Caj1 interaction with liposomes containing PA is modulated by pH and PE lipids and depends on two patches of positively charged residues near the C-terminus of the protein. The protein Caj1 is the first example of an Hsp40/J-domain protein with affinity for membranes and phosphatidic acid lipid specificity.
Structural insight into the Staphylococcus aureus ATP-driven exporter of virulent peptide toxins (2020). Science Advances
Zeytuni N, Dickey SW, Hu J, Chou HT, Worrall LJ, Alexander JAN, Carlson ML, Nosella M, Duong F, Yu Z, Otto M, Strynadka NCJ.
Staphylococcus aureus is a major human pathogen that has acquired alarming broad-spectrum antibiotic resistance. One group of secreted toxins with key roles during infection is the phenol-soluble modulins (PSMs). PSMs are amphipathic, membrane-destructive cytolytic peptides that are exported to the host-cell environment by a designated adenosine 5′-triphosphate (ATP)–binding cassette (ABC) transporter, the PSM transporter (PmtABCD). Here, we demonstrate that the minimal Pmt unit necessary for PSM export is PmtCD and provide its first atomic characterization by single-particle cryo-EM and x-ray crystallography. We have captured the transporter in the ATP-bound state at near atomic resolution, revealing a type II ABC exporter fold, with an additional cytosolic domain.
His-Tagged Peptidiscs Enable Affinity Purification of the Membrane Proteome for Downstream Mass Spectrometry Analysis (2020). Journal of Proteomic Research
John William Young, Irvinder Singh Wason, Zhiyu Zhao, David G Rattray, Leonard J Foster and Franck Duong Van Hoa
Characterization of the integral membrane proteome by mass spectrometry (MS) remains challenging due its high complexity and inherent insolubility. In a typical experiment, the cellular membranes are isolated, the proteins are solubilized and fractionated, and the detergent micelles are removed before MS analysis. Detergents are not compatible with mass spectrometry, however, and their removal from biological samples often results in reduced protein identification.
New Approach for Membrane Protein Reconstitution into Peptidiscs and Basis for their Adaptability to Different Proteins (2020). eLIFE
Gabriella Angiulli, Harveer Singh Dhupar, Hiroshi Suzuki, Irvinder Singh Wason,
Franck Duong Van Hoa and Thomas Walz
We introduced peptidiscs as a detergent substitute for stabilizing membrane proteins. This study presents ‘on-gradient’ reconstitution, a new method for gently reconstituting delicate membrane-protein complexes. We applied this method to the reaction center complexes from Rhodobacter sphaeroides, demonstrating that peptidiscs adapt to various shapes and sizes of transmembrane proteins. Using the ‘on-bead’ method, we successfully reconstituted Escherichia coli proteins MsbA and MscS, with peptidiscs maintaining their natural stability for high-resolution cryo-electron microscopy structures. The structures reveal that peptidisc peptides can surround transmembrane proteins in different configurations, explaining their stabilizing capacity. Our findings endorse peptidiscs as effective and simple alternatives to detergents for research involving membrane proteins.
PeptiQuick, a One-Step Incorporation of Membrane Proteins into Biotinylated Peptidiscs for Streamlined Protein Binding Assays (2020). JoVE
James Saville, Lucy Troman and Franck Duong
Membrane proteins, including transporters, channels, and receptors, constitute nearly one-fourth of the cellular proteome and over half of current drug targets. Yet, a major barrier to their characterization and exploitation in academic or industrial settings is that most biochemical, biophysical, and drug screening strategies require these proteins to be in a water-soluble state. Our laboratory recently developed the peptidisc, a membrane mimetic offering a “one-size-fits-all” approach to the problem of membrane protein solubility. We present here a streamlined protocol that combines protein purification and peptidisc reconstitution in a single chromatographic step. This workflow, termed PeptiQuick, allows for bypassing dialysis and incubation with polystyrene beads, thereby greatly reducing exposure to detergent, protein denaturation, and sample loss.
Profiling the E. coli Membrane Protein Interactome Captured in Peptidisc Libraries (2019). eLIFE
Michael Carlson, Greg Stacey, John Young, Irvin Wason, Zhiyu Zhao, David G Rattray, Nichollas Scott, Craig Kerr, Mohan Babu, Leonard J. Foster and Franck Duong
We apply the peptidisc for the capture of the Escherichia coli cell envelope proteome and its high-resolution fractionation in the absence of detergent. Using well-characterized membrane protein systems such as the SecY translocon, the Bam complex and the MetNI transporter, we find the peptidisc library very useful for identifying transient and novel protein interactions. Most of these interactions are largely undetected by standard detergent-based purification. The peptidisc workflow applied to the proteomic field is emerging as a promising novel approach to characterize membrane protein interactions under native expression conditions.
Investigating the stability of the SecA–SecYEG complex during protein translocation across the bacterial membrane (2019). The JBC
John Young and Franck Duong
SecA–SecYEG binding and dissociation events are important for efficient transport of the periplasmic protein proPhoA. A landmark previous study reported that ATP binding to SecA triggers a “power stroke,” resulting in forward movement of a polypeptide segment into the mouth of the SecYEG channel. Following ATP hydrolysis, when ADP is still bound to the enzyme, SecA adopts a conformation allowing the polypeptide to slide across the SecYEG channel until the next ATP- binding event. Instead, SecA rather operates as a “Brownian ratchet,” allowing substrates to passively diffuse within the SecY channel while the enzyme is in an ADP-state.
The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution (2018). eLIFE
Michael Luke Carlson, John William Young, Zhiyu Zhao, Lucien Fabre, Daniel Jun, Jianing Li, Jun Li, Harveer Singh Dhupar, Irvin Wason, Allan T Mills, J Thomas Beatty, John S Klassen, Isabelle Rouiller, and Franck Duong
Obtaining membrane bound proteins in a stable non-aggregated state remains a problem in the scientific community. Previous methods of using detergent micelles (small amphipathic molecules) are quite often detrimental to protein structure and activity, in addition to interfering with downstream analytical methods. However, using multiple copies of an amphipathic bi-helical peptide (here after termed NSPr) wrapping around a target membrane protein allows for its solubilization, retention of 3D structure and protein activity. Therefore allowing membrane proteins to be studied in a solubilized state.
Negative stain single-particle EM of the maltose transporter in nanodiscs reveals asymmetric closure of MalK and catalytic roles of ATP, MalE, and maltose (2017). The JBC
Lucien Fabre, Huan Bao, James Innes, Franck Duong and Isabelle Rouiller
The Escherichia coli MalE-MalFGK2 complex is one of the best characterized members of the large and ubiquitous family of ATP-binding cassette (ABC) transporters. It is composed of a membrane-spanning heterodimer, MalF-MalG; a homodimeric ATPase, MalK2; and a periplasmic maltose receptor, MalE. Opening and closure of MalK2 is coupled to conformational changes in MalF-MalG and the alternate exposition of the substrate-binding site to either side of the membrane. To further define this alternate access mechanism and the impact of ATP, MalE, and maltose on the conformation of the transporter during the transport cycle, we have reconstituted MalFGK2 in nanodiscs and analyzed its conformations under 10 different biochemical conditions using negative stain single-particle EM…..
Lipopolysaccharides promote binding and unfolding of the antibacterial colicin E3 rRNAse domain (2017). BBA-Biomembranes
Allan Mills and Franck Duong
Nuclease colicins are antibacterial proteins produced by certain strains of E. coli to reduce competition from rival strains. How exactly colicins traverse the cell envelope is not understood, yet this knowledge is important for the design of new antibacterial therapies. In this report, we discover that the cytotoxic rRNAse domain of colicin E3 is sufficient to inhibit cell growth. There is high affinity interaction (Kd ~1-2μM) between the rRNAse domain and lipopolysaccharides (LPS) which is important to destabilize the secondary structure of the toxin, which is expectedly crucial for transport through the membrane. The effect of LPS on binding and unfolding of ColE3 may be indicative of a broader role of LPS for transport of colicins in general.
Formation of a chloride-conducting state in the maltose ATP-binding cassette (ABC) transporter (2016). The JBC
Michael L. Carlson, Huan Bao and Franck Duong
ATP-binding cassette transporters use an alternating access mechanism to move substrates across cellular membranes. This mode of transport ensures the selective passage of molecules while preserving membrane impermeability. Through the use of a mutant that resides in intermediate conformations close to the transition state, we demonstrate that chloride conductance occurring, to a degree that can be large enough to compromise cell viability. We conclude that ABC transporters must stay away from these ion-conducting conformations to preserve the membrane barrier; otherwise, a few mutations that increase access to the ion-conducting states are enough to convert transporter to channel.
Sequential action of MalE and maltose allows coupling ATP Hydrolysis to translocation in the MalFGK2 transporter (2015). The JBC
Huan Bao, Kush Dalal, Eric Cytrynbaum and Franck Duong
ATP-binding cassette (ABC) transporters have evolved an ATP-dependent alternating-access mechanism to transport substrates across membranes. Despite important progress, especially in their structural analysis, it is still unknown how the substrate stimulates ATP hydrolysis, the hallmark of ABC transporters. In this study, we measure the ATP turnover cycle of MalFGK2 in steady and pre-steady state conditions. We show that (i) the basal ATPase activity of MalFGK2 is very low because the cleavage of ATP is rate-limiting, (ii) the binding of open-state MalE to the transporter induces ATP cleavage but leaves release of Pi limiting, and (iii) the additional presence of maltose stimulates release of Pi, and therefore increases the overall ATP turnover cycle. We conclude that open-state MalE stabilizes MalFGK2 in the outward-facing conformation until maltose triggers return to the inward-facing state for substrate and Pi release. This concerted action explains why ATPase activity of MalFGK2 depends on maltose, and why MalE is essential for transport.
FhuA interactions in a detergent-free nanodisc environment (2014). BBA-Biomembranes
Allan Mills, Hai-Tuong Le, James W. Coulton, and Franck Duong
Some metals and vitamins are critical for bacterial cell growth but their availability in the environment is limited. Gram-negative bacteria have evolved a set of outer membrane receptors to efficiently captureand transport these nutrients. Ferricrocin binds to FhuA to trigger the exposure of the TonB box to the periplasm. After recognition by TonB, the plug domain is pulled out of the β-barrel to allow the release of ferricrocin. Reconstitution of FhuA into nanodiscs binds TonB with a much lower affinity (~200 nM) than previously reported in detergent (~20 nM). Significantly, the binding critically depends on ferricrocin. FhuA in nanodiscs also forms a high-affinity binding site for colicin M.
Nucleotide-free MalK drives the transition of the maltose transporter to the inward-facing conformation (2014). The JBC
Huan Bao and Franck Duong
The complex MalFGK2 hydrolyzes ATP and alternates between inward- and outward-facing conformations during maltose transport. It has been shown that ATP promotes closure of MalK2 and opening of MalFG toward the periplasm. Yet, why the transporter rests in a conformation facing the cytosol in the absence of nucleotide and how it returns to this state after hydrolysis of ATP is unknown. The membrane domain MalFG may be naturally stable in the inward-facing conformation, or the ABC domain may catalyze the transition. We address this question by analyzing the conformation of MalFG in nanodiscs and in proteoliposomes. We find that MalFG alone exists in an intermediate state until MalK binds and converts the membrane domain to the inward-facing state.
SecYEG activates GTPases to drive the completion of cotranslational protein targeting (2013). The JCB
David Akopian, Kush Dalal, Kuang Shen, Franck Duong, and Shu-ou Shan
A conserved protein-conducting channel, SecYEG in bacteria or Sec61p in eukaryotes, is the point of convergence of post- and co-translational protein targeting pathways and mediates the translocation or integration of newly synthesized proteins. Extensive work on the Sec pathway showed that SecYEG and pre-proteins stimulate SecA’s ATPase activity and activate it to drive the translocation of pre-proteins into the periplasm. During targeting, SRP and FtsY form a heterodimer in which both proteins undergo a series of conformational changes, including the early, closed, and activated states, that culminate in reciprocal GTPase activation.
ATP alone triggers the outward-facing conformation of the maltose ABC transporter (2013). The JBC
Huan Bao and Franck Duong
The maltose transporter MalFGK(2) is a study prototype for ABC importers. During catalysis, the MalFG membrane domain alternates between inward and outward facing conformations when the MalK dimer closes and hydrolyzes ATP. Because a rapid ATP hydrolysis depends on MalE and maltose, it has been proposed that closed liganded MalE facilitates the transition to the outward facing conformation. Here we find that, in contrast to the expected, ATP is sufficient for the closure of MalK and for the conversion of MalFG to the outward facing state. The outward facing transporter binds MalE with nanomolar affinity, yet neither MalE nor maltose is necessary or facilitates the transition. Thus, the rapid hydrolysis of ATP observed in the presence of MalE and maltose is not because closed liganded MalE accelerates the formation of the outward facing conformation. These findings have fundamental implications for the description of the transport reaction.
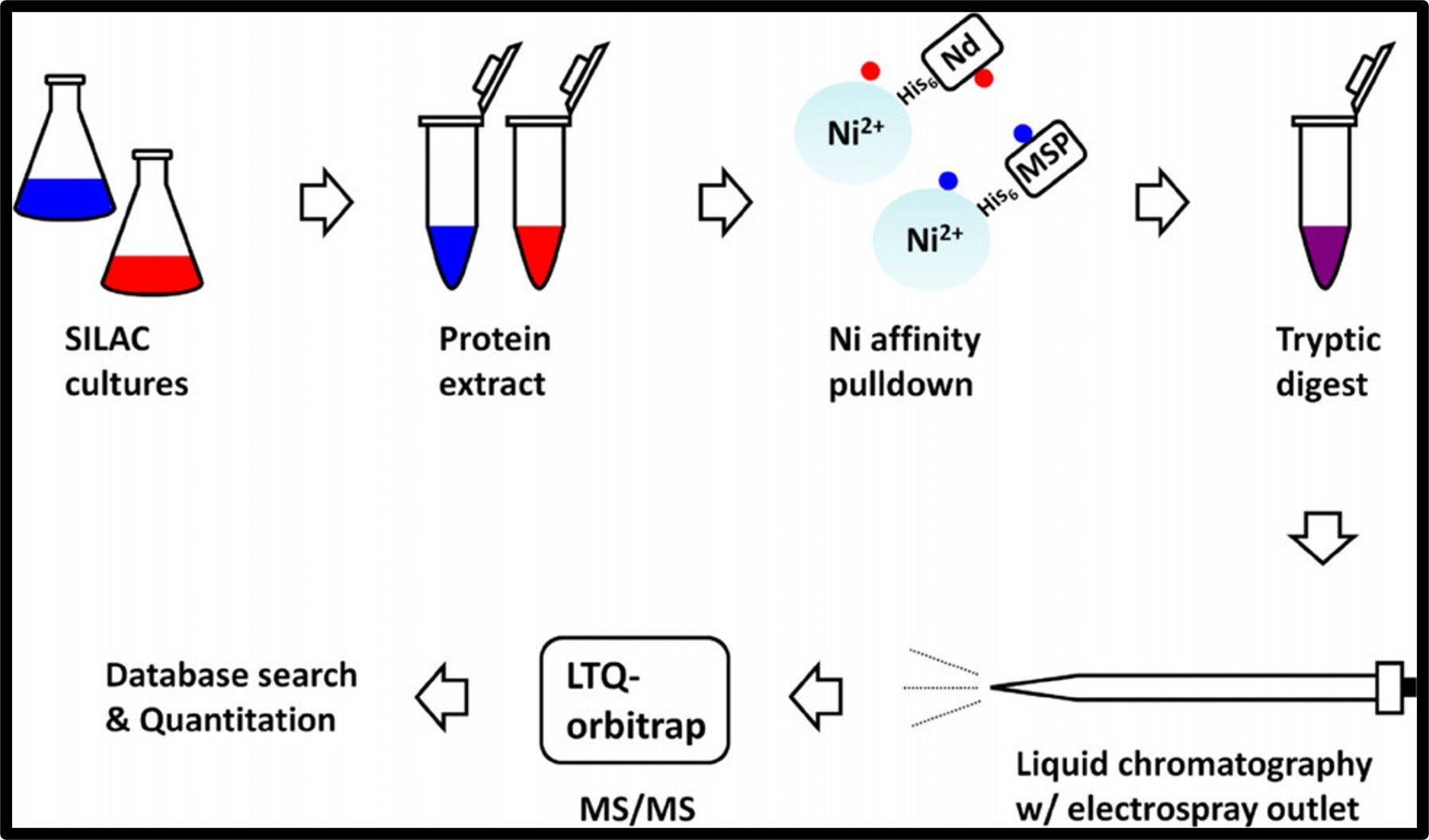
Nanodiscs and SILAC-based mass spectrometry to identify a membrane protein interactome (2012). The JPR
Xiao X. Zhang, Catherine S. Chan, Huan Bao, Yuan Fang, Leonard J. Foster and Franck Duong
Integral membrane proteins are challenging to work with biochemically given their insoluble nature; the nanodisc circumvents the difficulty by stabilizing them in small patches of lipid bilayer. Here, we show that nanodiscs combined with SILAC-based quantitative proteomics can be used to identify the soluble interacting partners of virtually any membrane protein. As a proof of principle, we applied the method to the bacterial SecYEG protein-conducting channel, the maltose transporter MalFGK(2) and the membrane integrase YidC. In contrast to the detergent micelles, which tend to destabilize interactions, the nanodisc was able to capture out of a complex whole cell extract the proteins SecA, Syd, and MalE with a high degree of confidence and specificity. The method was sensitive enough to isolate these interactors as a function of the lipid composition in the disc and the culture conditions.
Two copies of the SecY channel and acidic lipids are necessary to activate the SecA translocation ATPase (2012). PNAS
Kush Dalal, Catherine S. Chan, Stephen G. Sligar and Franck Duong
The nanodisc is a discoidal particle (~ 10-12 nm large) that trap membrane proteins into a small patch of phospholipid bilayer. The nanodisc is a particularly attractive option for studying membrane proteins, especially in the context of ligand-receptor interactions. The method pioneered by Sligar and colleagues is based on the amphipathic properties of an engineered highly a-helical scaffold protein derived from the apolipoprotein A1. The hydrophobic faces of the scaffold protein interact with the fatty acyl side-chains of the lipid bilayer whereas the polar regions face the aqueous environment. Analyses of membrane proteins in nanodiscs have significant advantages over liposome because the particles are small, homogeneous and water-soluble. In addition, biochemical and biophysical methods normally reserved to soluble proteins can be applied, and from either side of the membrane.
The SecY complex: conducting the orchestra of protein translocation (2011). TICS
Kush Dalal and Franck Duong
Like the conductor of an orchestra, the Sec protein translocation channel is the platform needed to bring together the many different players required for the constitutive and obligatory process of protein transport. This conserved membrane channel, termed SecY in bacteria and Sec61 in eukaryotes, creates a ubiquitous protein-conducting pathway by which thousands of newly synthesized polypeptides make their way through the lipid bilayer. The channel is not a simple passive pore, however; it displays remarkable complexity by interacting with numerous soluble partners, including SecA, Syd, FtsY and the ribosome in bacteria.
The SecY complex forms a channel capable of ionic discrimination (2011). EMBO Rep
Kush Dalal and Franck Duong
Protein translocation across the bacterial membrane occurs at the SecY complex or channel. The resting SecY channel is impermeable to small molecules owing to a plug domain that creates a seal. Here, we report that a channel loosely sealed, or with a plug locked open, does not, however, lead to general membrane permeability. Instead, strong selectivity towards small monovalent anions, especially chloride, is observed. Mutations in the pore ring-structure increase both the translocation activity of the channel and its ionic conductance, however the selectivity is maintained. The same ionic specificity also occurs at the onset of protein translocation and across the archaeal SecY complex. Thus, the ion-conducting characteristic of the channel seems to be conserved as a normal consequence of protein translocation. We propose that the pore ring-structure forms a selectivity filter, allowing cells to tolerate channels with imperfect plugs.
Previous Publications
Bao H, Dalal K, Cytrynbaum E, Duong F. (2015) Sequential action of MalE and maltose allows coupling ATP Hydrolysis to translocation in the MalFGK2 transporter. J Biol Chem. jbc.M115.671826.
Duong F (2014). Capturing the bacterial holo-complex. Proc Natl Acad Sci U S A. 111:4739-40. 41.
Bao H, Duong F (2014). Nucleotide-free MalK drives the transition of the maltose transporter to the inward-facing conformation. J Biol Chem. 289:9844-51.
Mills A, Le HT, Coulton JW, Duong F (2014). Evaluating the FhuA interactions in a detergent-free nanodisc environment. Biochim Biophys Acta. 1838:364-71.
Bao H, Duong F (2013). Phosphatidylglycerol directs binding and inhibitory action of EIIAGlc on the maltose transporter. J Biol Chem. 288:23666-74.
Bao H, Dalal K, Wang V, Rouiller I, Duong F (2013). The maltose ABC transporter: action of membrane lipids on the transporter stability, coupling and ATPase activity. BBA Biomembranes. 1828:1723-1730.
Akopian D, Dalal K, Shen K, Duong F, Shan S-o (2013). SecYEG activates GTPases to drive the completion of cotranslational protein targeting. J Cell Biol. 200:397-405.
Bao H, Duong F (2013) ATP alone triggers the outward-facing conformation of the maltose ABC transporter. J Biol Chem. 288:3439-48.
Bao H, Duong F, Chan CS (2012). A step-by-step method for the reconstitution of an ABC transporter into nanodisc lipid particles. J Vis Exp, e3910. *corresponding author.
Bao H, Duong F (2012). Discovery of an auto-regulation mechanism for the maltose ABC transporter MalFGK2. PloS ONE 7:e34836. Important new findings for an otherwise well known transporter .
Dalal K, Chan CS, Sligar SG, Duong F (2012). Two copies of the SecY channel and acidic lipids are necessary to activate the SecA translocation ATPase. Proc Natl Acad Sci U S A 109:4104-4109. This work is a succesful the application of the nanodiscs to isolate and study membrane protein oligomers.
Fonseca BD, Diering GH, Bidinosti MA, Dalal K, Alain T, Balgi AD, Forestieri R, Nodwell M, Rajadurai CV, Gunaratnam C, Tee AR, Duong F, Andersen RJ, Orlowski J, Numata M, Sonenberg N, Roberge M (2012). Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem 287:17530-45.
Zhang XX, Chan CS, Bao H, Fang Y, Foster LJ, Duong F (2012). Nanodiscs and SILAC-based mass spectrometry to identify a membrane protein interactome. J Proteome Res 11:1454-1459.
Dalal K, Duong F (2011). The SecY complex: conducting the orchestra of protein translocation. Trends in Cell Biology 21:506-514. Review.
Gold VA, Robson A, Bao H, Romantsov T, Duong F, Collinson I (2010). The action of cardiolipin on the bacterial translocon. Proc Natl Acad Sci U S A 107:10044-10049.
Dalal K, Bao H, Duong F (2010). Modulation of the SecY channel permeability by pore mutations and trivalent cations. Channels 4:83-96.
Dalal K, Duong F (2009). Reconstitution of the SecY complex in Nanodiscs. In Protein Secretion. Ed. E. Economou, Methods in Molecular Biology 619:145-156.
Dalal K, Duong F (2009). The SecY complex forms a channel capable of ionic discrimination. The EMBO Rep 10:762-768.
Dalal K, Nguyen N, Alami M, Tan J, Moraes TF, Lee WC, Maurus R, Sligar SS, Brayer GD, Duong F (2009). Binding, structure and activity of Syd, a SecY interacting protein. J Biol Chem 284:7897-7902. The details of the work were highlighted by the editor of Nature Structural & Molecular Biology 16, pp106 (2009).
Duong F (2007). “ Cell biology: Fraternal twins ” Nature News & Views, 446:741-743. Commentary.
Gold V, Duong F, Collinson I (2007). The bacterial protein translocation reaction. Mol Membr Biol 24:387-394. Review.
Alami M, Dalal K, Lelj-Garolla B, Sligar S and Duong F (2007). Nanodiscs unravel the stoichiometry of the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J 26:1995-2004. The principal discovery was highlighted by the editor of Science 316, pp174 (2007).
Maillard A, Lalani S, Silva F, Belin D, Duong F (2007). Deregulation of the SecYEG translocation channel upon removal of the plug domain. J Biol Chem 282:1281-1287.
Tam P, Maillard A, Chan K, Duong F (2005). Investigating the SecY-plug movement at the SecYEG translocation channel. EMBO J 24:3380-3388.